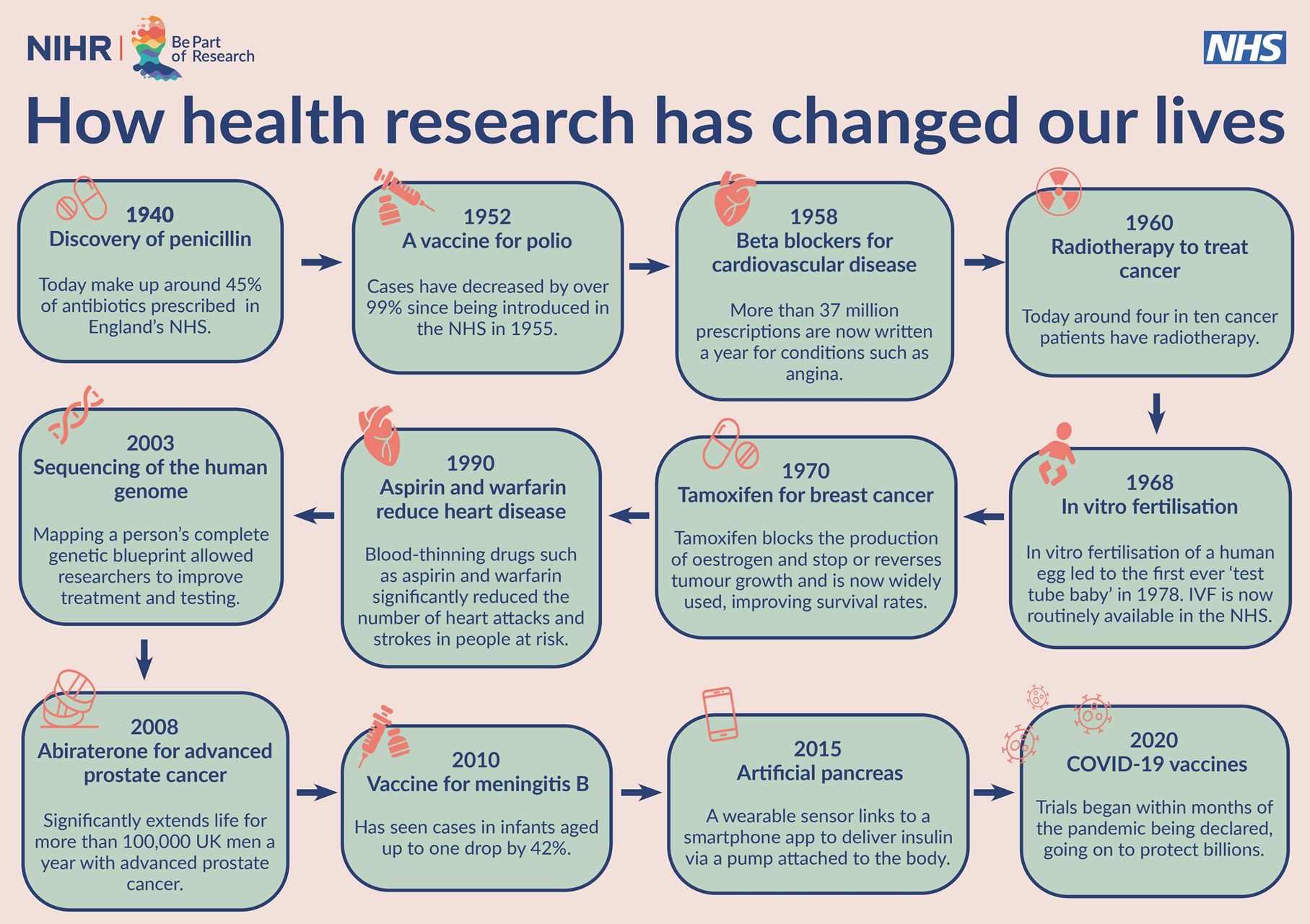
Research

Research at the Health Centre
The Health Centre has been supporting research for some years. The Practice supports important national studies that are organised by other researchers.
Taking part in research
If you meet the entry criteria for a particular research study that we are running at this practice, your doctor or nurse may discuss this with you. Alternatively, we may send you information via email or SMS if we think you might be suitable for a new study.
If you are interested in finding out more about that study, we will ask for your permission to pass your name and contact details on to the relevant study team. For some studies, you might be asked to return a reply slip direct to the study team. The research team will then make contact with you directly to tell you more about the study and what it involves.
Bicester Health Centre Active Studies
Click the links below to find out more about the current studies being undertaken at Bicester Health Centre.
TIGER (Trial of food allergy (IgE) tests for Eczema Relief) -
for children aged 3 months - 2 years.
RESTED (Randomised Evaluation of Sleep Treatment to Ease Depression) - Adults
ARI (Acute Respiratory Infection) -
ages 60+
T2T (Gout) -
ages 18+
THARROS (COPD) -
ages 40-80 with a COPD diagnosis who have never taken an inhaled corticosteroid for their COPD.
Surviving Crying -
for parents aged 18+ struggling with a crying baby.
ECRAID-Prime -
ages 18+, for people with cold/flu like symptoms to trial a nasal spray.


With regard to our research activity, please be assured that:
- Participation in research is entirely voluntary and you have the right to say ‘No’. Nobody will put pressure on you to take part in research if you do not wish to. You do not have to give us a reason if you decide not to take part.
- Your care and your relationship with your doctor or nurse will not be affected in any way if you decide not to take part in a research study.
- You will always receive clear information about what taking part in a research study would involve. The practice will usually provide you with a patient information sheet; then, if you agree to take part, the study team will explain the study to you in more detail and you will have the opportunity to ask questions about it.
- Nobody from outside this practice will be given your contact details or have access to your medical records without your prior consent. If you do agree to take part in a study, you will be asked to sign a consent form – this will clearly state which parts of your notes (if any) may be looked at for the purposes of the research.
- You will not be asked to take part in a large number of studies. Most researchers are very specific about the criteria that people need to meet in order to enter their study. Usually this means that only a relatively small number of patients at the practice will be suitable for any one study.
What if I don’t want to get involved in research?
We recognise that some people may not want to receive information about research studies by email or SMS. If you do not want to be contacted about research studies that we may run at the practice in the future, please let us know. If you change your mind at a later date, you can still opt back in at any time.
Anonymous Data Sharing For Research
The Clinical Practice Research Datalink (CPRD) is a large database of anonymised electronic medical records collected at Primary Care clinics throughout the UK. Through the electronic clinical system we use to record medical notes, a completely anonymous (i.e. no identifiable patient details are shared at all) extract is taken which is used for medical research. Clinical data in CPRD are catalogued for medical conditions, symptoms and important background information together with a wide variety of clinical measurements and prescribed medications. The extract includes the following information:-
|
Patient |
age, sex, registration date when entering the practice, and date when leaving the practice |
|
Medical |
medical diagnoses, date of diagnosis, and location (e.g., GP’s office, hospital, consultant) of the event and an option for adding free text; |
|
Therapy |
all prescriptions along with the date issued, formulation, strength, quantity, and dosing instructions, indication for treatment for all new prescriptions (inferred from cross reference to medical events on the same date), and events leading to withdrawal of a drug or treatment. |
|
Additional Health Data (AHD) |
vaccinations and prescription contraceptives; miscellaneous information such as smoking, height, weight, immunizations, pregnancy, birth, death, and laboratory results. |
|
Postcode Variable Indicators (PVI) |
postcode linked area based socio-economic, ethnicity and environmental indices |
|
|
date, time and duration of consultation |
|
|
gender and roles of staff who entered the data |
You do not have to participate in this research and we will happily block your record from being included; please speak to the reception team if you would like to be excluded.
Page created: 21 December 2023
